Validating Singapore patents in Cambodia
Validation or re-registration of a Singapore patent in Cambodia means the recognition, on request, of the effects of the Singapore patent in Cambodia, which enables a right holder of a Singapore patent to obtain his/her patent in Cambodia within a shorter timeframe. We provide below some remarks on the matter for your reference:
| No. | Titles | Remarks |
| 1 | Signing date | • On 20 January 2015, the Ministry of Industry & Handicraft of the Kingdom of Cambodia (“MIH”, currently the Ministry of Industry, Science, Technology & Innovation, or “MISTI”) and the Intellectual Property Office of Singapore (“IPOS”) entered into the Memorandum of Understanding on the Co-operation in Industrial Property (“MOU”). • As a follow up to MOU, MIH adopted Prakas (declaration) No. 182 MIH/2016 dated 27 July 2016 on the re-registration of Singapore patents in Cambodia. IPOS has issued a Guideline to Re-register a Singapore Patent in Cambodia. • On 14 January 2020, MIH and IPOS entered into Renewal of the Memorandum of Understanding. |
| 2 | Effective date | • MOU comes into effect since the date of its signature by both parties, i.e. 20 January 2015, and can be renewed upon the mutual consent of both parties. • MOU covers industrial designs apart from inventions/utility models. |
| 3 | Benefits | • A Singapore patent validated in Cambodia will have the same validity as a corresponding Cambodian patent. • Procedure for validating a Singapore patent in Cambodia is quite simple and fast (where the patentee of a Singapore valid patent files a request for the validation, MIH will conduct only formality examination and issue a patent certificate). • Such validation may save some kinds of fees and accelerate the grant of a patent in Cambodia. |
| 4 | Requirements | The following requirements must be met to validate a Singapore patent in Cambodia: • The Singapore patent must be in force at the time of lodgment of reregistration request. • The Singapore patent must have a filing date on or after 11 February 2003 (i.e. the date on which the Law on Patent of Cambodia took effect). • The Singapore patent is granted and in force. • Required documents: (i) Notarized Power of Attorney; (ii) Certified copy of the granted Singapore patent; (iii) Certified copy of the final specifications of the Singapore patent; (iv) Certified Khmer translations of the abstract and final specifications of the Singapore patent within 6 months of the lodgment date. • The Singapore patent must meet the Cambodian requirements for the patent protected matters. |
| 5 | Exclusions | • Per Article 136 of the Cambodian Law on Patents, Utility Models and Industrial Designs, pharmaceutical products are excluded from patent protection. Cambodia currently benefits from the World Trade Organization waiver that allows Least Developed Countries (LDCs) to avoid granting and enforcing IP rights on pharmaceutical products until the end of 2033. This waiver would also apply to Singapore patents that provide protection for pharmaceutical products, for which validation is sought in Cambodia. |
| 6 | Flowchart for validation | Download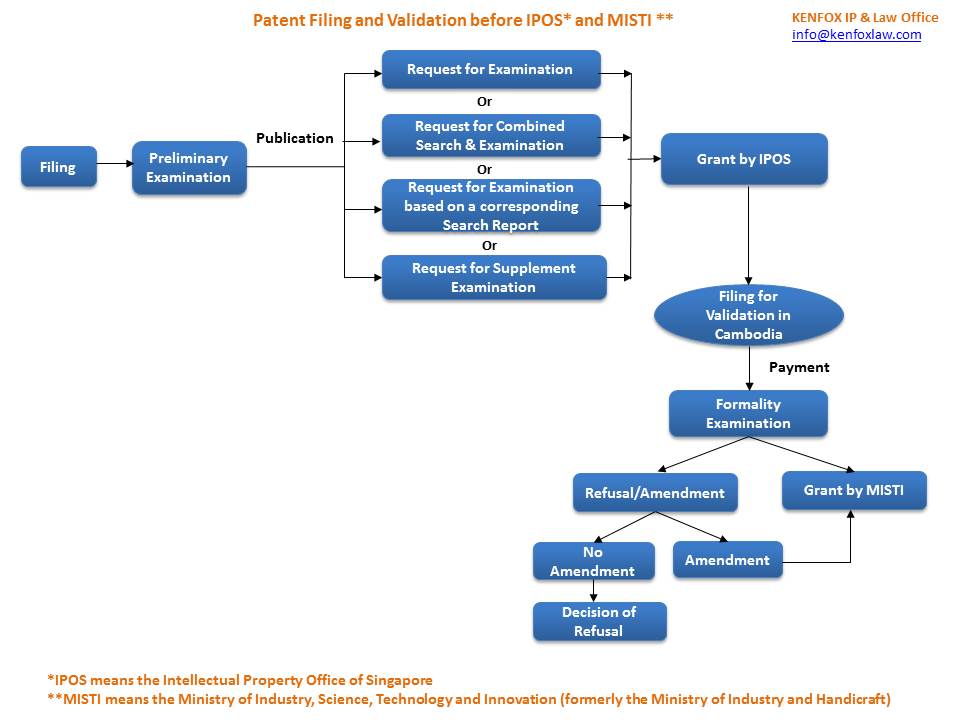 |
| 7 | Others | • There is no deadline to validate a Singapore patent in Cambodia. In other words, a request for validation can be filed any time during the validity of a Singapore patent. • The validation of the Singapore patent is subject to payment of administrative fees prescribed in the joint Prakas on Provision of Public Services and Transitional Fines of MISTI and the Ministry of Economy and Finance. • The term of a patent validated before MISTI based on a Singapore patent under MOU is 20 years computed from the filing date of the Singapore patent application. • Where the Singapore patent is terminated due to failure to pay annuity fees, the patent granted in Cambodia remains valid as long as the annuity requirements for the Cambodian patent are met. |
Related Article:

